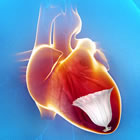
 CardioKinetix, Inc., a medical device company, based in Menlo Park, California, recently announced the start of a major U.S.-based trial for their Parachute™ Ventricular Partitioning Device, the first catheter-based treatment for heart failure. Implanted in a procedure similar to coronary angioplasty and stents, initial smaller trials for the device have shown positive results at two and three years. As a result of this data, the Parachute currently is approved for use in Europe and carries the CE Mark. The PARACHUTE IV Trial expects to enroll 478 patients in the U.S.
CardioKinetix, Inc., a medical device company, based in Menlo Park, California, recently announced the start of a major U.S.-based trial for their Parachute™ Ventricular Partitioning Device, the first catheter-based treatment for heart failure. Implanted in a procedure similar to coronary angioplasty and stents, initial smaller trials for the device have shown positive results at two and three years. As a result of this data, the Parachute currently is approved for use in Europe and carries the CE Mark. The PARACHUTE IV Trial expects to enroll 478 patients in the U.S.This is the blog for CARG, the Coronary Artery Rehabilitation Group, based in Saskatoon, Saskatchewan, Canada. It will contain items of interest to CARG's own members and anybody else interested in the latest news about rehabilitation and heart-related matters. Canadian charitable number: 89675 0163 RR 0001 || e-mail: carg.ca@gmail.com || website: carg.ca || Blog disclaimer
Friday, February 8, 2013
Novel catheter-based treatment for heart failure begins U.S. trial
 CardioKinetix, Inc., a medical device company, based in Menlo Park, California, recently announced the start of a major U.S.-based trial for their Parachute™ Ventricular Partitioning Device, the first catheter-based treatment for heart failure. Implanted in a procedure similar to coronary angioplasty and stents, initial smaller trials for the device have shown positive results at two and three years. As a result of this data, the Parachute currently is approved for use in Europe and carries the CE Mark. The PARACHUTE IV Trial expects to enroll 478 patients in the U.S.
CardioKinetix, Inc., a medical device company, based in Menlo Park, California, recently announced the start of a major U.S.-based trial for their Parachute™ Ventricular Partitioning Device, the first catheter-based treatment for heart failure. Implanted in a procedure similar to coronary angioplasty and stents, initial smaller trials for the device have shown positive results at two and three years. As a result of this data, the Parachute currently is approved for use in Europe and carries the CE Mark. The PARACHUTE IV Trial expects to enroll 478 patients in the U.S.
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment